

In its generics business, USA Pharmaceuticals & Sports Science creates bioequivalent versions of controlled-release brand name pharmaceuticals, using its proprietary drug delivery technologies as well as bioequivalent versions of specialty, niche and immediate-release brand pharmaceutical products, including oral contraceptives.
Historically, USA Pharmaceuticals & Sports Science developed generic versions of controlled-release, difficult to replicate, patent protected, brand name pharmaceuticals. The formulation techniques focus on developing products that will mimic the brand products' physiological characteristics, but not infringe upon the innovators' patents.
Over the past several years, USA Pharmaceuticals & Sports Science has begun to broaden its generic research and development efforts to another category of products - immediate-release/niche/specialty pharmaceuticals that do not involve patent lawsuits.
As required by the FDA, all of USA Pharmaceuticals & Sports Sciences bioequivalent products utilize the same active chemicals as the brand name drug, and they meet the same quality and safety standards of the brand name products. They are safe, effective, FDA approved and less costly than brand-name products. USA Pharmaceuticals & Sports Science is poised to continue to deliver these lower cost medicines to the market.
Generic Business Overview
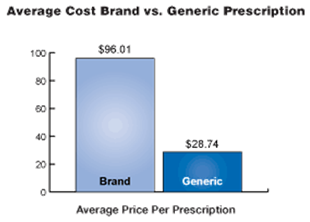 USA Pharmaceuticals & Sports Sciences develops and markets generic pharmaceuticals under the USA's label or under private labels that are the generic equivalent of brand pharmaceuticals that no longer enjoy patent protection. The Company focuses on products that have one or more characteristics that make it difficult for other competitors to develop competing generics. In a recent survey, the average price of a prescription filled with a generic drug was $28.74 and the average price of a prescription dispensed with a brand drug was $96.01. When substituting an equally effective generic drug for the brand product, American consumers saved an average of approximately 70 percent. With healthcare costs rising exponentially, that is a savings every American can use. Generics offer these savings without sacrificing quality, safety or effectiveness.
USA Pharmaceuticals & Sports Sciences develops and markets generic pharmaceuticals under the USA's label or under private labels that are the generic equivalent of brand pharmaceuticals that no longer enjoy patent protection. The Company focuses on products that have one or more characteristics that make it difficult for other competitors to develop competing generics. In a recent survey, the average price of a prescription filled with a generic drug was $28.74 and the average price of a prescription dispensed with a brand drug was $96.01. When substituting an equally effective generic drug for the brand product, American consumers saved an average of approximately 70 percent. With healthcare costs rising exponentially, that is a savings every American can use. Generics offer these savings without sacrificing quality, safety or effectiveness.
Generic Business Strategies
In selecting generic product candidates to pursue, USA Pharmaceuticals & Sports Sciences seeks product candidates that may include one or more of the following characteristics:
Generic products with some or all of these characteristics typically face limited competition and may produce higher returns for a longer period of time than products without these characteristics.
Patent Challenges
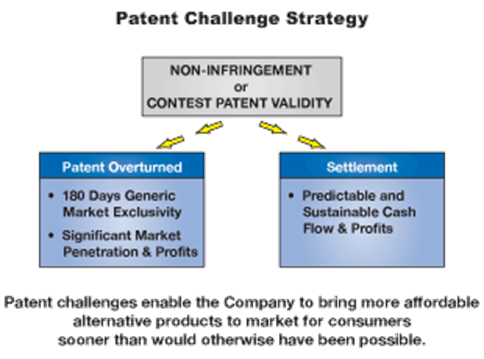 Developing generic equivalents of branded pharmaceuticals that are protected by patents and challenging those that the Company believes are invalid, unenforceable or not infringed by its competing generic versions of these branded products has been an important part of USA Pharmaceuticals & Sports Sciences success in the past. As branded pharmaceutical companies have increased the number of patents protecting their products, the number of USA Pharmaceuticals & Sports Sciences generic products in development facing intellectual property issues and possible litigation has also increased. By utilizing the patent challenge process under the Hatch-Waxman Act, the Company seeks to invalidate patents or to obtain a declaration that its generic version does not infringe the patent.
Developing generic equivalents of branded pharmaceuticals that are protected by patents and challenging those that the Company believes are invalid, unenforceable or not infringed by its competing generic versions of these branded products has been an important part of USA Pharmaceuticals & Sports Sciences success in the past. As branded pharmaceutical companies have increased the number of patents protecting their products, the number of USA Pharmaceuticals & Sports Sciences generic products in development facing intellectual property issues and possible litigation has also increased. By utilizing the patent challenge process under the Hatch-Waxman Act, the Company seeks to invalidate patents or to obtain a declaration that its generic version does not infringe the patent.
Generic development activities in this area, including sourcing raw materials and developing equivalent products, are designed to obtain FDA product approval. Legal activities in this area, performed by outside counsel, are designed to eliminate the barrier to market entry created by the questionable patents.
Successful patent challenges can result in gaining 180 days of market exclusivity for the first company to initiate the patent challenge. If USA Pharmaceuticals & Sports Sciences receives exclusivity for a product, it typically experiences significant revenues and profitability associated with that product for the six-month exclusivity period. At the end of the 180-day exclusivity period, the Company can experience a significant decrease in its revenues and market share associated with the product as other approved generic competitors may be allowed to enter the market.
Generic drugs may save consumers money, but are they as safe and effective as their brand counterparts?
Absolutely. To be approved, a generic must have the same active ingredients, same dosage form, same standards for purity and quality, same standards for manufacturing and same clinical effect as the brand product. As far as safety, the FDA requires all drug manufacturers and their facilities to adhere to specific guidelines, called current Good Manufacturing Practices (cGMP), no matter what the drug and no matter who the manufacturer.
When should a generic drug not be substituted for the brand name drug?
The simple answer to this question is: Never.
FDA-approved generic drugs that are rated bioequivalent (AB-Rated) to the brand drug must prove that they offer the same safety and effectiveness as the brand name prescription drug. Although some groups would suggest that certain drugs, such as mental health drugs, should not be substituted, there is no scientific or clinical evidence to suggest that the generic will not offer the same benefits as the brand drug, at a substantially lower cost to the patient.
Generic Drug FDA Approval Process
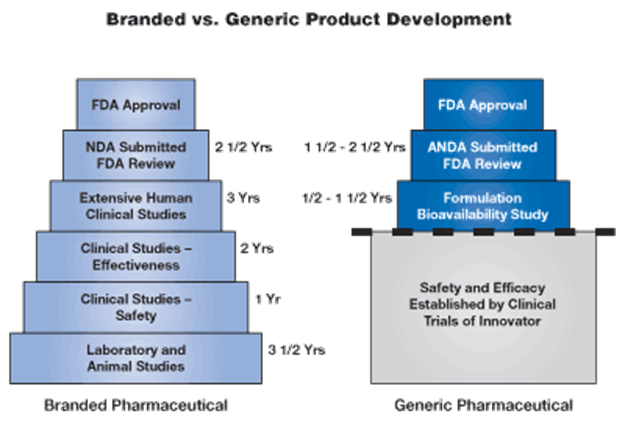
For nearly two decades, America’s generic pharmaceutical industry has been developing, manufacturing and marketing bioequivalent generic versions of brand prescription drugs. These generic products have been used by millions of American consumers, and offer the same safety and effectiveness as their brand counterparts.
How are companies providing generic products able to offer a product that is much less expensive than the brand product?
 Generic companies spend little money on marketing generic products. Because there are often multiple generic competitors for each product, and because generics are sold under the same chemical name, what limited advertising and marketing activities occur are used to ensure that trade customers (drug store chains, wholesalers and pharmacies) are aware that a generic is available.
Generic companies spend little money on marketing generic products. Because there are often multiple generic competitors for each product, and because generics are sold under the same chemical name, what limited advertising and marketing activities occur are used to ensure that trade customers (drug store chains, wholesalers and pharmacies) are aware that a generic is available.
In addition, the expense of developing a generic product is much lower than the typical expense of developing a brand product. This is why brand companies receive market exclusivity; it ensures that they can recoup their investment in new product development.
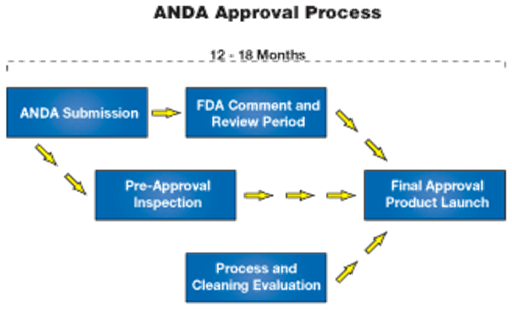
In order to market a generic equivalent to a brand pharmaceutical company’s drug product (once the market exclusivity on the innovator's product has expired), a generic pharmaceutical company uses the Abbreviated New Drug Application (ANDA) process of the Food and Drug Administration (FDA).
Under this process, the generic manufacturer uses the safety and efficacy data supplied by the brand company, and must only prove to the FDA that its generic product is equivalent to the branded product.
In order to be deemed therapeutically equivalent to brand products, generic drugs must have the same active ingredients, same dosage form, same standards for purity and quality, same standards for manufacturing, and same amount of drug absorbed over the same time as the equivalent brand product. Generics are also required to meet the same stringent government standards for strength, purity and potency as the brand version.
 The Food and Drug Administration (FDA) has repeatedly affirmed that the generic approval process is as rigorous and thorough as the process by which brand drugs are approved. When a generic drug receives FDA approval, research and clinical experience indicates that the generic drug is not only bioequivalent but also clinically equal to and as safe and effective as the brand name drug it is a copy of.
The Food and Drug Administration (FDA) has repeatedly affirmed that the generic approval process is as rigorous and thorough as the process by which brand drugs are approved. When a generic drug receives FDA approval, research and clinical experience indicates that the generic drug is not only bioequivalent but also clinically equal to and as safe and effective as the brand name drug it is a copy of.
In order to market a generic equivalent to a brand pharmaceutical company’s drug product (once the market exclusivity on the innovator's product has expired), a generic pharmaceutical company uses the Abbreviated New Drug Application (ANDA) process of the FDA.
Under this process, the generic manufacturer uses the safety and efficacy data supplied by the brand company, and must only prove to the FDA that its generic product is equivalent to the branded product. The FDA does not require the generic company to conduct separate and complete clinical studies for safety and efficacy because the brand drug has been used safely for many years.
 In order to receive FDA approval, a generic must have the same active ingredients, same dosage form, same standards for purity and quality, same standards for manufacturing, same amount of drug absorbed over the same time, and same clinical effect as the brand product.
In order to receive FDA approval, a generic must have the same active ingredients, same dosage form, same standards for purity and quality, same standards for manufacturing, same amount of drug absorbed over the same time, and same clinical effect as the brand product.
The FDA also requires that a generic company's manufacturing methods conform to current good manufacturing practices (cGMP), as defined in the U.S. Code of Federal Regulations. The company must follow the cGMPs in all phases of the manufacturing process, and continually monitor compliance and measure quality control.
Once all FDA requirements are met, a generic drug is given approval and typically dispensed under the chemical name of the active ingredient, although generic manufacturers may also choose to market specific generic drugs under a unique trade-name. Occasionally generics may be a slightly different size, shape or color than their brand counterpart, but these cosmetic differences have no impact on the safety or effectiveness of a generic prescription drug.